The EPiC trial

This clinical trial aims to demonstrate the clinical and cost-utility of High-Flow Therapy (HFT), assessing the viability of home HFT as a suitable therapy for patients that have previously been hospitalised due to severe Chronic Obstructive Pulmonary Disease (COPD) exacerbation.
Study objectives*
The main objective of the trial is to investigate if home High-Flow Therapy (HFT) improves 12-month admission-free survival times, following a severe exacerbation of COPD requiring hospitalisation.
Patient population
Main inclusion criteria
• Patients admitted to hospital with an exacerbation of COPD
• Age 40-80 years with smoking history >10 pack years
• Patient’s forced expiratory volume in 1 second (FEV1) is <80% predicted
• Patient’s FEV1/forced vital capacity (FVC) < 0.7
Study details
Study type
Prospective, multicentre, open-label, randomised, controlled trial with parallel groups.
Primary investigators
Prof Nicholas Hart
Dr Patrick Murphy
Lane Fox Clinical Respiratory Physiology, Research Centre, St Thomas Hospital
Sponsor
Guy’s and St Thomas’ Hospital NHS Foundation Trust
Study design
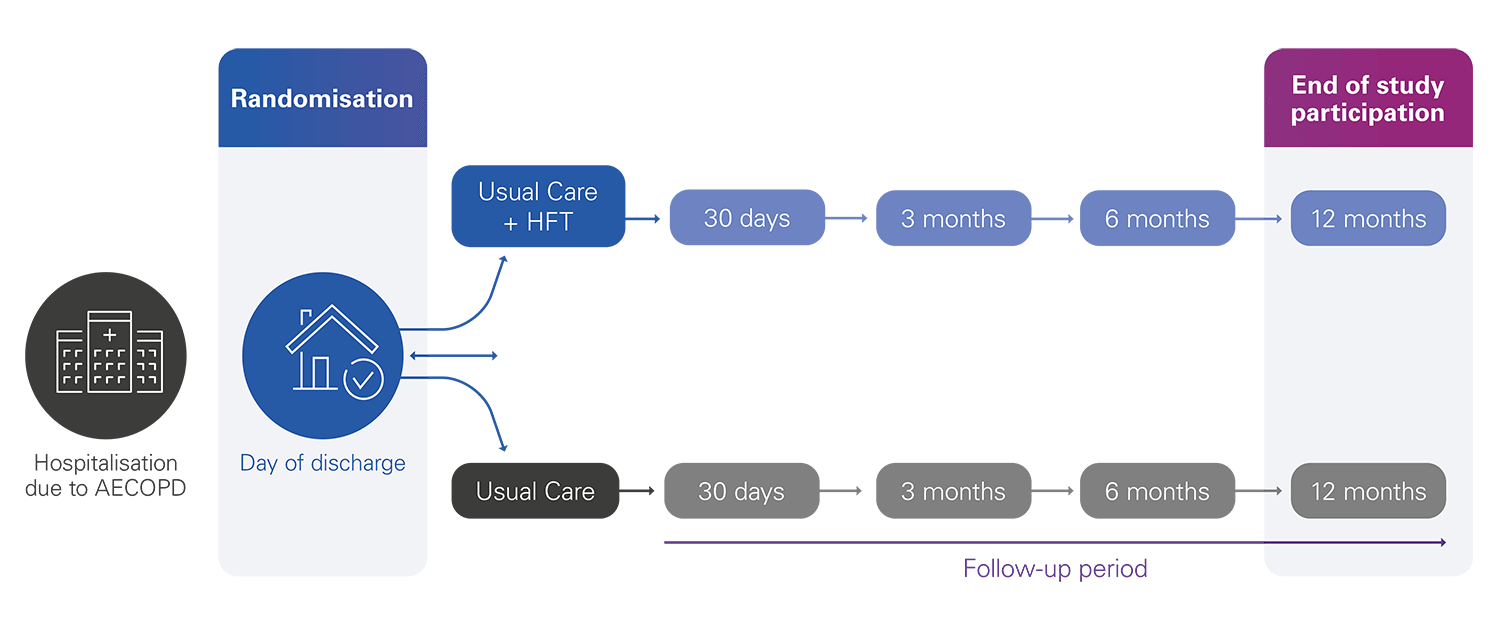
502 patients randomised into two groups:
• Intervention: Usual care + Home HFT
• Control: Usual care
The enrolment period will last 24 months, with a follow-up period of 12 months, for a total study duration of 36 months.
The study endpoints
Primary endpoint:
12-month all cause admission-free survival
Secondary endpoints:
• Patient reported outcome measures (CAT, PSQI, EQ-5D-5L)**
• Physiological effects of HFT
• Cost utility analysis
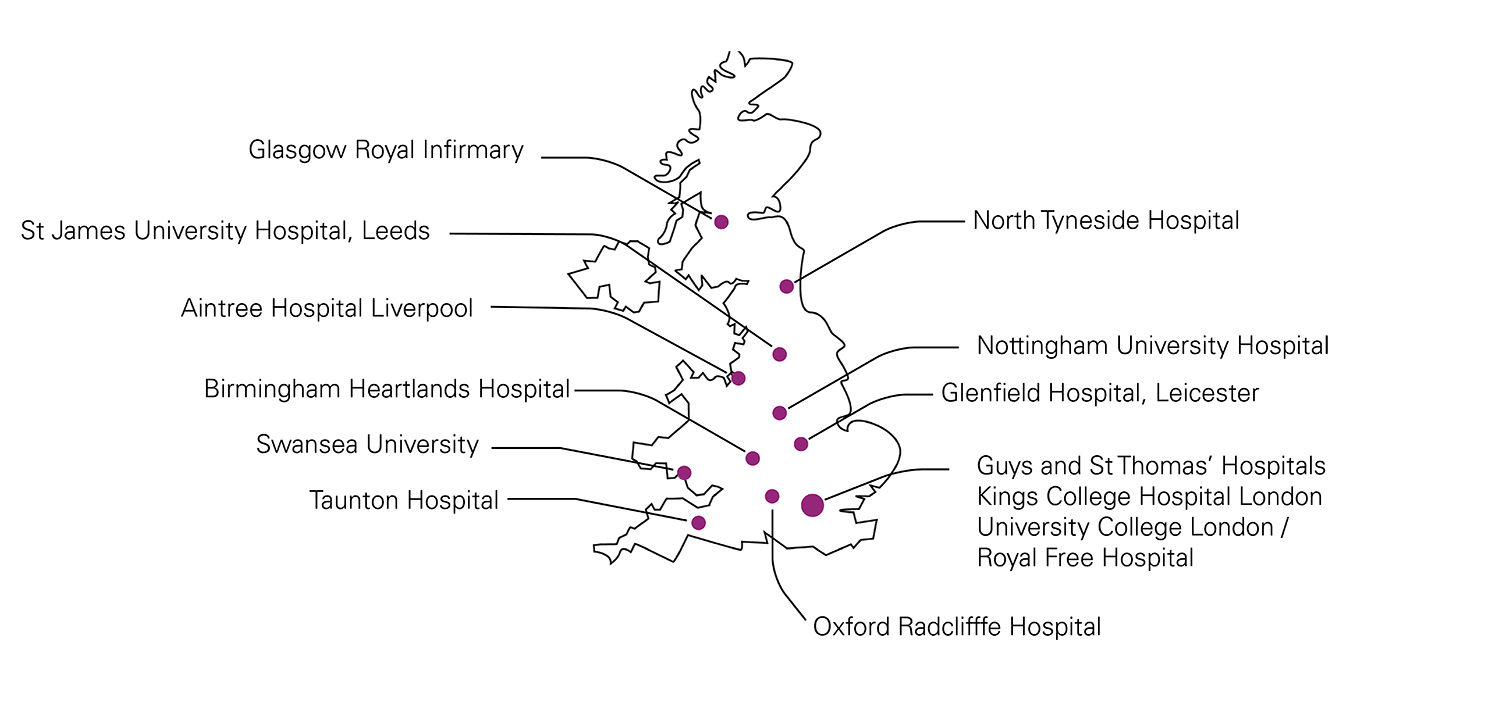
14 proposed centres
Hear from the investigators
Learn more about this clinical trial from the investigator’s webpage.
Learn more: Home HFT for COPD

Home high-flow therapy for COPD
Learn how HFT could be indicated for home use in COPD patients experiencing secretion management issues.

Webinars on home high-flow therapy
Experts explain the evidence for using HFT in different indications, discuss current research gaps and explore the potential benefits of this emerging therapy.
This content is intended for health professionals only
* For more information visit the investigator’s research page https://www.kcl.ac.uk/research/epic-hft
** CAT, computerised adaptive tests; PSQI, Pittsburgh sleep quality index; EQ-5D-5L, EuroQol 5-dimension, 5-level quality of life questionnaire.